New technology developed to efficiently produce embryoids
A team of University of Michigan and University of California, San Francisco researchers developed a new technology able to efficiently produce human embryo-like, also called embryoids. The research was published in Nature on Sept. 11.
Other scientists have created embryoids in the past, which are made by coaxing human stem cells to behave and organize into structures similar to ones found in very early human embryos. The researchers specifically use pluripotent stem cells, which are able to differentiate into a variety of cell types.
The new technology uses a microfluidics device in which scientists first insert pluripotent stem cells and then add chemicals that stimulate the cells to develop into human embryo-like structures.
Jianping Fu, associate professor of mechanical engineering, led the team that has worked on the project since 2015. He said his team was able to generate embryoids with about 5 to 10 percent efficiency a few years ago, while the new technology is about 95 percent efficient.
Yi Zheng, a post-doctoral research fellow in Fu’s lab, said he felt encouraged by all the attention the research received.
“I published quite a few research papers before, but none of them generated so many interests and attentions from both academia and the public,” Zheng said in an email to The Daily. “It is very interesting to read about other’s comments from totally different perspectives. I feel quite proud that my research is found useful and important by the top scientists in the field, all my hard work finally pays off.”
LSA junior Gillian Rubenstein said she read the paper after finding a link to it in a newsletter from Nature. Rubenstein, who is interested in gender and health, said she’s excited for the possible implications the research has and the fact it’s emerged in the reproductive field.
“(Stem cell research) doesnt seem like, so far, it’s gotten into the reproductive field, so I thought it was really exciting that potentially we could create embryos out of stem cells,” Rubenstein said.
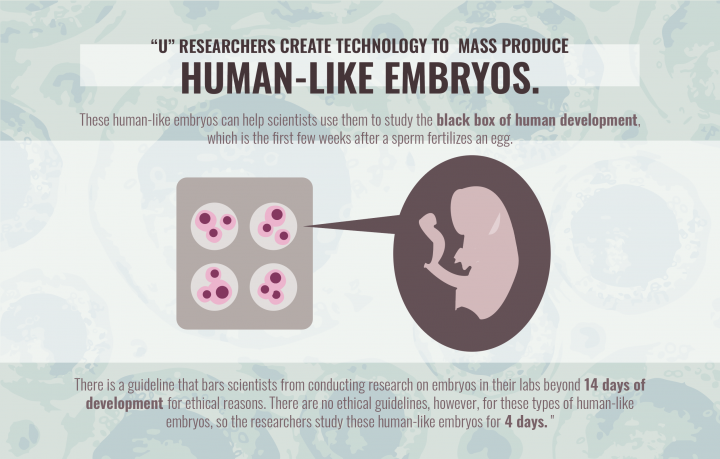
Fu said the new technology could aid scientists in learning more about what he calls the “black box of human development.” This is the first few weeks after a sperm fertilizes an egg and the embryo implants into the wall of the uterus, called the peri-implantation stage.
“During this period, many miscarriages can happen and serious birth defects can form as well,” Fu said. “I think we are very excited and very hopeful that this system … has generated a reliable, experimental platform for answering fundamental questions about human development.”
Scientists have a good idea of what happens for the nine months of pregnancy, but have had trouble understanding the peri-implantation stage because the embryo isn’t easily accessible during this period. The 1984 Warnock rule has also made it difficult to research peri-implantation — the Warnock rule, also known as the 14-day rule, bars researchers from studying human embryos beyond 14 days of development for ethical reasons.
Fu said there are no ethical guidelines for embryoids yet. He hopes embryoids aren’t subjected to the 14-day rule because they do not contain certain cell types, so they are unable to develop into a baby. The lab has followed the 14-day rule so far by only culturing the embryoids for four days, Fu said.
“By the (four day) point, in fact, the human embryo-like structures will start to disassemble and collapse,” Fu said. “We are still thinking about ways how we could continuously, properly culture them so to continuously model and downstream human developmental events, but I think that’s the future.”
Fu said some of the attention from the media has been inaccurate. He criticized many in the media who have described the technology as being able to “mass produce” embryoids. He argued the system is scalable and efficient, but the system is not designed to mass produce these types of structures.
“I think we should be cautious saying that,” Fu said. “I don’t think that’s very precise. It’s really just the system we are generating … has very high efficiency, which means, if needed, we can generate a lot of human embryo-like structures, but not necessarily massively producing such structures.”
Fu also mentioned embryoids shouldn’t be called “synthetic” or “artificial” human embryos because it may lead to misinformation about the team’s research and possibly cause damage to the field.
“That could easily cause a lot of misconceptions … when you say that,” Fu said. “People already have the impression of, ‘Oh, this is almost equivalent to a human embryo,’ which is not true.”
Although some bioethicists have criticized Fu’s research, Rubenstein said it seems Fu’s team followed many of the current ethical procedures.
“Because there’s no ethical guidelines, it’s hard to say, you know, ‘Is this OK or is this not OK,’” Rubenstein said. “I think it’s encouraging that they’re acting on the safe side … as long as they’re following standard protocols that already exist, the guidelines are going to have to grow to match the technology.”
Fu said human embryo-like could be used in the future to screen drugs because there is a relationship between late onset diseases and early development. Fu mentioned many current drugs pregnant women take have unknown effects on embryo development.
Zheng agreed with Fu. He said he hopes the embryoids are used as model systems to uncover important information about early human development.
“The system is a powerful tool, opening up a door to previously inaccessible period of human development,” Zheng said. “A lot of existing hypotheses in human embryology can be conveniently tested without using human embryos or fetal tissues. It can also be further developed into a useful drug screening platform for preventing pregnancy loss and birth defects.”
Though there aren’t any ethical guidelines for the research Fu is conducting, he said the experiments conducted were approved by ethical committees at the University. Fu added he hopes guidelines are eventually created.
“All the protocols we use to generate such human embryo-like structures using human stem cells have already been approved by the ethical committees at the University,” Fu said. “As I mentioned, we understand there are ethical considerations in such research, so we are hoping that scientists and all the stakeholders … can talk and figure out guidelines to guide the proper, continuous progress of this very exciting research.”
Fu’s research dealing with human embryo-like structures has not received any federal support, and it remains unclear how federal funding agencies will be willing to support such emerging research given the Dickey-Wicker amendment. Fu said his team relied on funding from the University, including the mechanical engineering department, Mcubed and a partnership fund between the University and Cambridge University.
Fu hopes to continue work on this project, specifically creating more reliable human embryo-like systems in the future.
“There are many, many different directions that can be pursued from here,” Fu said. “I’m very interested in thinking about how we can develop strategies to allow continuous, proper development of such human embryo-like structures, which means that we want to push further so that allows us to generate some reliable model systems to even understand downstream developmental events.”